Abstract
Bispecific T-cell engagers (BiTE® antibody constructs) represent a novel immunotherapeutic strategy relying on the recruitment of T cells against tumor cells independent of TCR specificity. In Acute Myeloid Leukemia (AML), CD33 represents a suitable target antigen with high expression levels in >90 % of primary AML samples (Krupka et al, 2014). A CD33-BiTE® antibody construct (AMG 330) was developed mediating cytotoxicity against primary AML in vitro although to a variable degree (Krupka et al, 2016). Several parameters have been identified which modulate AMG 330-mediated cytotoxicity, including CD33 expression level as well as effector to target cell (E:T) ratio. However, the exact mechanism of T-cell activation through BiTE® antibody constructs is only partly understood. Physiological T-cell activation is based on engagement of the T-cell receptor complex together with costimulatory molecules whereas the absence of positive costimulation leads to T-cell anergy.
In line with this concept, we hypothesized that BiTE®-mediated cytotoxicity requires positive costimulatory signals on the target cells for T-cell activation. We hypothesize that the ratio of costimulatory and coinhibitory molecules on AML cells determines the susceptibility to AMG 330-mediated cytotoxicity independent of target antigen expression level.
A stable expression system was established utilizing murine Ba/F3 cells expressing human CD33 ± CD80 ± CD86 ± PD-L1. Co-cultures of Ba/F3 constructs and T cells were performed in presence of AMG 330 or a control BiTE® (cBiTE®) (5 ng/ml). For some experiments, T cells were separated into naive (CD45RA+/CCR7+) vs memory (CD45RADIM) cells using fluorescence-activated cell sorting. After 3 days, specific lysis was determined by flow cytometry and calculated as % specific lysis = 100 × (1 - live CD33+ cellsAMG 330 / live CD33+ cellscBiTE). T-cell proliferation was defined as number of CD2+ cells on day 3 compared to day 0.
The expression pattern of CD33, CD80, CD86 and PD-L1 on primary AML cells was evaluated by specific fluorescence intensity (SFI) using multiparameter flow cytometry. A sample was considered positive at an SFI of > 1.5. Characterized primary AML patient samples were used in a long-term culture assay to determine the influence of the checkpoint molecule expression profile on AMG 330-mediated cytotoxicity.
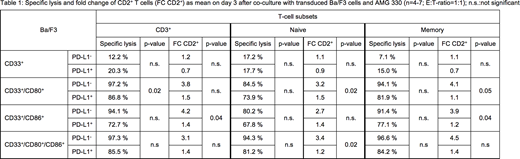
CD33 single positive Ba/F3 cells were not lysed upon the addition of AMG 330 and allogeneic T cells. Cytotoxicity could be restored by expression of CD80, CD86 and CD80+CD86 with following tendency: CD80+CD86 >> CD80 > CD86 (see table 1). There was a direct correlation of T-cell proliferation to AMG 330 mediated cytotoxicity. Memory T cells showed increased cytotoxicity compared to naive T cells against the different Ba/F3 cell lines. The influence of co-inhibition was investigated by additionally transducing PD-L1 into the different Ba/F3 cells. This led to a reduced AMG 330-mediated cytotoxicity in all PD-L1 expressing Ba/F3 cells (Table 1). This was accompanied by a reduction in T-cell proliferation.
Looking at the expression profile of CD80 and CD86 in primary AML samples, we observed expression of CD80 in 7/123 and of CD86 in 188/226 of cases (respectively 5.7 % and 83.2 %).
When comparing AMG 330-mediated cytotoxicity against primary AML cells for patient pairs with similar CD33 expression levels, a higher CD86/PD-L1 ratio led to an increased AMG 330-mediated cytotoxicity compared to patient samples with a lower CD86/PD-L1 ratio (exemplary data: SFI CD33+: 81.7; SFI-ratio CD86/PD-L1: 4; specific cytotoxicity: 64.2 % vs. SFI CD33+: 89.5; SFI-ratio CD86/PD-L1: 15.9; specific cytotoxicity: 96.4 %).
In summary, this data supports the hypothesis that AMG 330-mediated cytotoxicity and T-cell proliferation are influenced by the ratio of costimulatory and coinhibitory molecules on AML cells. Our data supports the notion that the checkpoint profile on AML, rather than one molecule by itself, determines T-cell response to AMG 330. Prospective analyses in clinical trials are needed to validate the relevance of checkpoint molecules on target cells as a predictive biomarker for response.
Marcinek:AMGEN Research Munich: Research Funding. Brauchle:AMGEN Inc.: Research Funding. Kischel:AMGEN: Employment. Kufer:AMGEN Research Munich: Employment. Subklewe:Pfizer: Membership on an entity's Board of Directors or advisory committees; Roche AG: Research Funding; AMGEN: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees; Gilead Sciences: Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal